In Which of the Following Reactions Would Energy Be Created
Energy can be defined as the capacity to supply heat or do work. Reaction occurring during the process photosynthesis is also an endothermic reaction in which glucose and oxygen are obtained as product from carbon dioxide and water by absorbing the energy from sunlight.

Energy Diagram Overview Parts Expii
A Energy is neither created nor destroyed in chemical reactions.
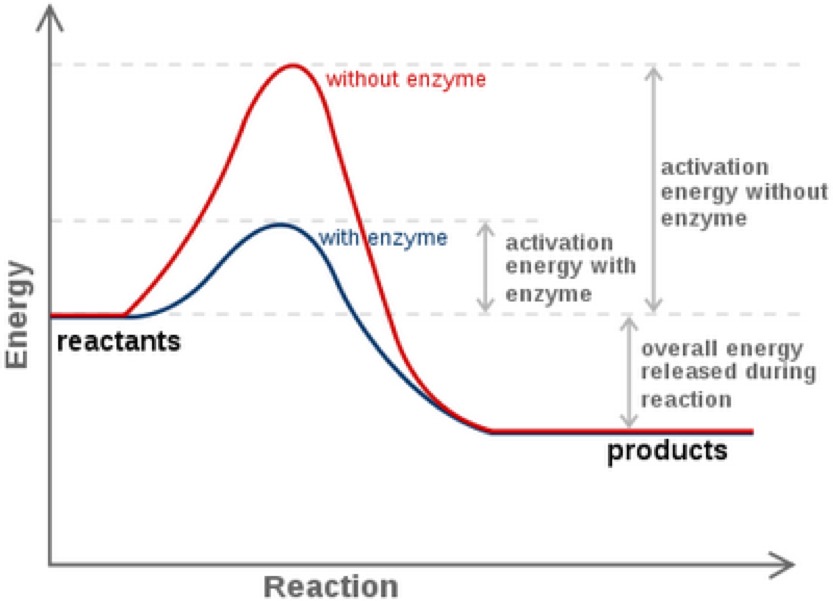
. REDOX reactions consist of two types of reactions. Organisms use energy to survive grow respond to stimuli reproduce and for every type of biological process. Identify the missing reactant or product in each of the following equations.
This will theoretically burn the highest number of calories. Where energy is treated as a reactant is classified as an endothermic reaction. Energy cannot be created or destroyed but can be changed from on form to another.
All of the above are true. Temperature of the surrounding also increases. The potential energy stored in molecules can be converted to chemical energy which can.
In endothermic reaction the energy is absorbed from the surroundings. Therefore higher is the concentration that is more will be the number of reactants present in the chemical reaction. This energy can be quantified on the basis of the amount of fuel used.
A change the amount of energy needed for a reaction. Energy cannot be created or destroyed meaning that the total amount of energy in the universe has always been and will always be constant. Above reaction takes place in the aqueous mediumAfter the reaction mixtures becomes hot.
On the other hand a reaction that requires energy ie. According to collision theory more is the number of collisions occurring in a chemical reaction more faster will be the rate of reaction. Thermal energy Energy that results from atomic and molecular motion.
Law of entropy transformation. Glucose Oxygen Carbon dioxide Water Energy as 30-32 ATP The body releases carbon dioxide and water in this process. The bonds in the reactants are broken and the bonds of the products are formed.
One type of work w is the process of causing matter to move against an opposing force. For example we do work when we inflate a bicycle tirewe move matter the air in the pump against the opposing force of the air already in the tire. A common example of energy transfer that we see in everyday life is the transfer of kinetic energythe energy.
Energy can be transferred from the system to its surroundings or vice versa but it cant be created or destroyed. Energy is released as a result of which of the following chemical reactions. How much energy is required to change the temperature of 215 g.
Neutralization reaction between acid and base. Chemistry questions and answers. Not all of the captured solar energy becomes carbohydrates.
They consume carbon dioxide and produce oxygen as a waste product. 6CO 2 6H 2 O energy - C 6 H 12 O 6 6O 2. C change the type of substrate that binds the enzymes active site.
Chemical reactions that can generate electricity are called REDOX reactions. B prevent the substrate from binding the enzymes active site. Results from atomic and molecular motion.
Since every chemical change involves a gain or loss of energy. Remember that all chemical reactions involve a change in the bonds of the reactants. In an endothermic reaction heat is absorbed.
Where Δ Energy. Energy is absorbed by the reaction. C Endothermic processes transfer heat from the surroundings into the system.
One is called reduction while the other is called oxidation. The first law of thermodynamics can be captured in the following equation which states that the energy of the universe is constant. For an Endothermic reaction the change in enthalpy delta H is.
Hence they are not oxidation-reduction reactions. During photosynthesis plants use energy originally from sunlight to convert carbon dioxide gas CO 2 into sugar molecules like glucose. However this does not mean that energy is immutable.
Under other physiological conditions the. In an exothermic reaction heat is released. It can change form and even transfer between objects.
For example when solid dry ice vaporizes physical change carbon dioxide molecules absorb energy. Energy is a property of objects which can be transferred to other objects or converted into different forms but cannot be created or destroyed. The exothermic reactions are the reactions in which energy is released The released energy can be in the form of heat or light.
In an Exothermic reaction the energy of the reactants is ____ than the energy of the products. When you combine the Red part of reduction to the Ox part of oxidation you will get. Ozone can be created from oxygen gas with an input of energy via the following reaction.
The faster the motion the greater the thermal energy. The forms of energy include thermal energy radiant energy electrical energy nuclear energy and chemical energy Figure 51 Forms of Energy. When leaf cells photosynthesize they use solar energy to form carbohydrate molecules from carbon dioxide and water.
When liquid water becomes ice energy is released. Include any coefficient. 302 g 늑 203 g If the equilibrium constant K is 112 10-54 for this reaction at a particular temperature and 021 310 10-2 M at equilibrium what is 03 in M to two decimal places at equilibrium.
This reaction is summarized as. C 6 H 12 O 6. The faster the motion the higher the thermal energy.
A reaction which releases energy is exothermic. D change the type of product produced in the reaction. B Potential energy is the energy associated with motion.
Terms in this set 28 Exothermic Reaction. D Increasing the thermal energy of a gas increases the motion of its atoms e Energy is the capacity to do work. A reaction which absorbs energy is endothermic.
CH 4g 2O2g CO2g 2H 2Og Δ. REDOX reactions occur in the batteries you usually buy to power your electronic devices.
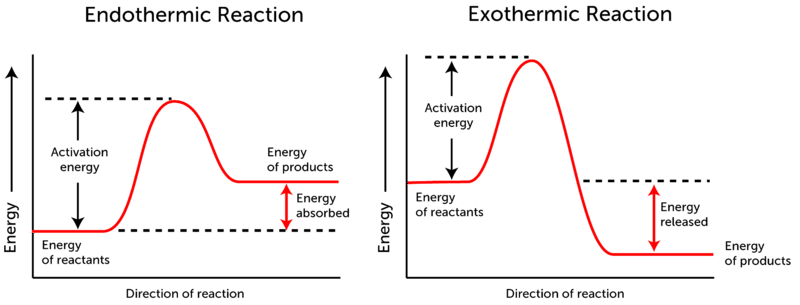
Chemical Reactions And Energy Ck 12 Foundation
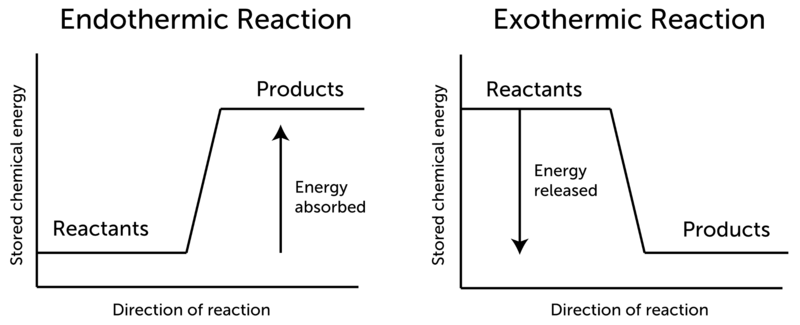

No comments for "In Which of the Following Reactions Would Energy Be Created"
Post a Comment